Receptors and cellular communication
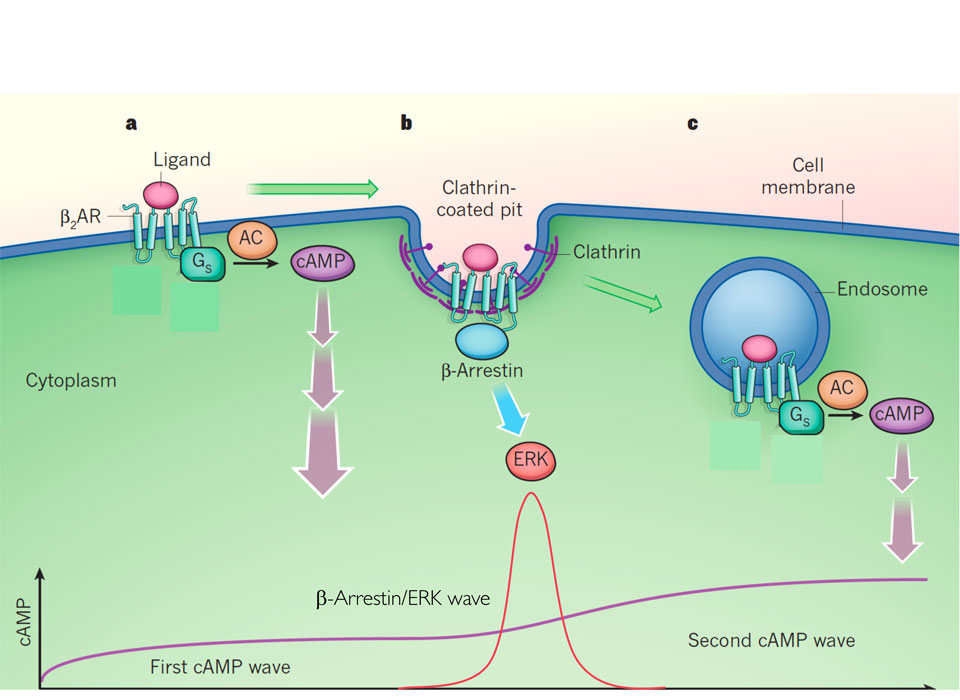
G-Protein-coupled receptors - their activation, signals and cellular localization
Adapted from Lohse&Calebiro, Nature 495, 457-458
Overview
Our group focuses on the mechanisms of activation and signaling of G-protein coupled receptors (GPCR), and subsequent downstream events which lead to alterations in cell function.
G protein-coupled receptors (GPCRs) are a large family of approximately 800 members, which include receptors for light, taste and smell, but also many receptors for transmitters and hormones. They are also the most important group of targets for drugs, for example beta blockers, antihistamines and opiates.
All GPCRs share common structural features, presumably also common activation mechanisms, and they all signal via heterotrimeric G proteins (Gαβγ) and – at later points upon activation, via β-arrestins. We use a variety of biochemical and pharmacological methods as well as new microscopic techniques to study the mechanisms of their function and their potential to serve as targets for innovative drug therapies.
We work closely together with the Receptor Signalling Lab at the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC), where large parts of our group are active. Some topics and methods are preferably established there, others at ISAR Bioscience in Planegg.
Topics
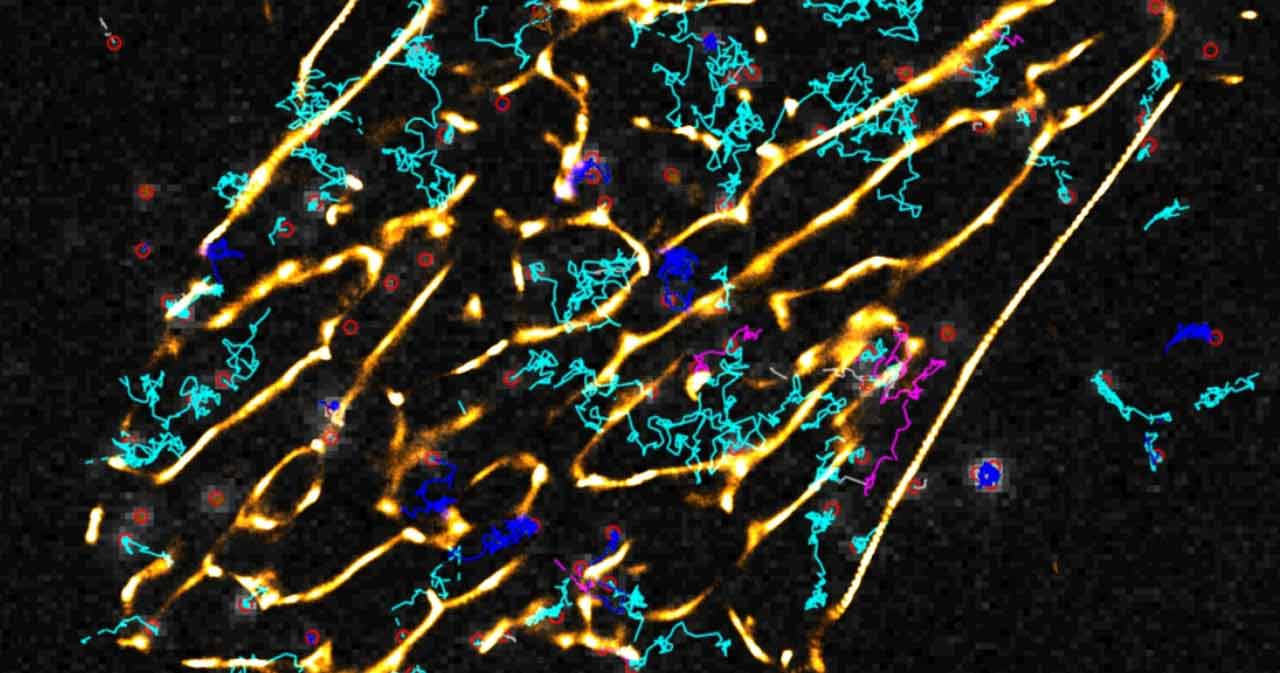
Where, when and how fast do receptors get activated?
GPCR activation is triggered by binding of a ligand (e.g. adrenaline, glutamate) to a specific site of a receptor. This is followed by a conformational change in the receptor, which enables it to bind to and activate G-proteins. We have developed methods to monitor these signalling events via fluorescence microscopy, and we aim to study where and when in a cell these signals occur. We use techniques to trigger receptor activation by light to monitor signaling with sub-millisecond resolution. We are also working on techniques to adapt such measurements to high throughput screening in order to search for compounds with unusual signalling properties, which might make them new classes of drugs with only a subset of effects compared to conventional drugs.
Does cAMP come in nanodomains?
Cyclic adenosine monophosphate (cAMP) is a second messenger downstream of GPCR activation, which plays an important role in many physiological processes, ranging from regulation of heartbeat and force to synaptic plasticity. Although cAMP appears to be a freely diffusible substance, recent evidence suggests that it may act in cells in a highly localized manner. We are attempting to visualize such local effects and are searching for mechanisms that might lead to the postulated nano-compartments. To do so, we use models ranging from the design and expression of specific cAMP sensor proteins to studies in transgenic fly and mouse models, where we attempt to image local cAMP signals.
How and why receptors make oligomers?
GPCRs can form supramolecular complexes (i.e. di-/oligomers) on the cell surface. However, their size, nature and dynamics as well as their physiological and pharmacological implications are still largely unknown. We focus on investigating the dimerization of receptors (especially adrenergic, opiate and chemokine) on the surface of intact cells as well as evaluating the role of dimerization on receptor activation and downstream signalling. We do so by a variety of biochemical and microscopic techniques, notably brightness analysis and single particle microscopy.
Methods
Epifluorescent, confocal, super-resolution
To track receptors or effector proteins we use fluorescent labels of different nature: fluorescent proteins, antibodies, non-organic chemicals, etc. A broad range of microscope designs is available in our lab, including confocal microscope, total internal reflection fluorescence microscope, custom-built microscope for photolysis. Microscopes are equipped with the newest generation of photodetectors and cameras, providing high temporal and spatial resolution of measurements.
Electronic energy transfer through nonradiative dipole–dipole coupling
Major biophysical tools used in our lab are Förster Resonance Energy Transfer (FRET) and Bioluminescence Resonance Energy Transfer (BRET). They allow to measure distances between domains and motifs within or between proteins, providing information about receptor conformations, G-protein activation, second messengers concentration, etc.
Luminescence-based drug screening in microtiter plates
Using BRET-based assays adapted to microtiter plates we can measure large numbers of compounds and determine their effects on receptors and signal cascades.
Biochemical assays to study protein and nucleic acid functions
We have developed numerous biochemical and functional assays to characterize proteins and nucleic acids involved in signaling processes.
Recent Key Publications
Publikationen AG Lohse in PubMed